Contents
What is Surface Tension?
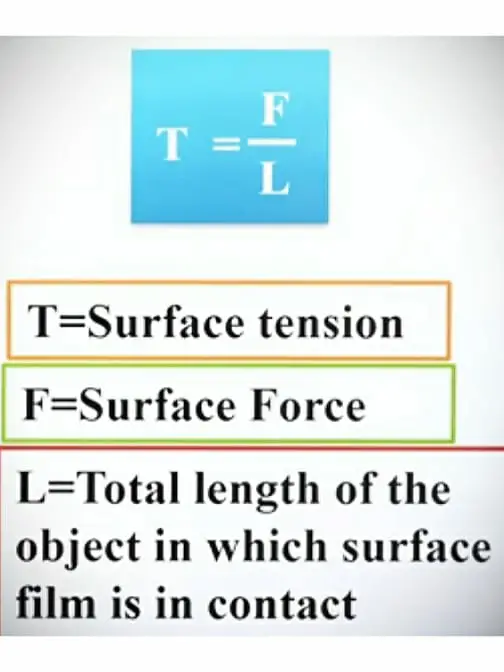
Surface tension is defined as the force per unit length acting at right angles to an imaginary line drawn on the free surface of the liquid.
OR
Surface tension is also defined as the tensile forces acting on the surface of a liquid in contact with a gas or on the surface between two immiscible liquids such that the contact surface behaves like a membrane under tension.
Unit of Surface Tension
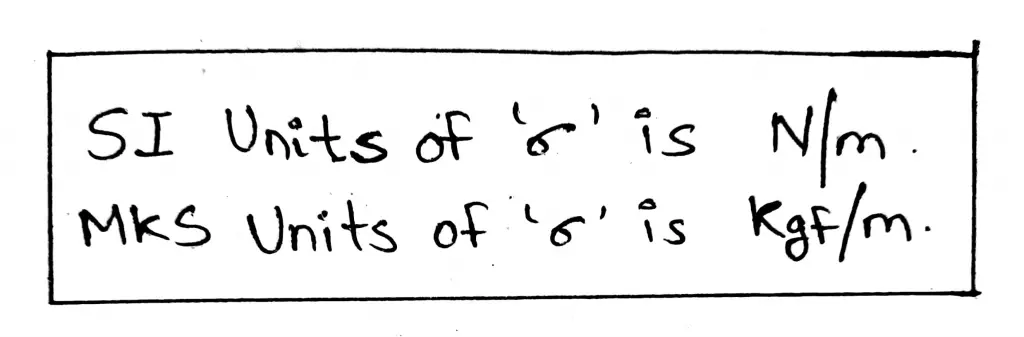
The unit of surface tension is denoted by the Greek letter (uppercase Σ, lowercase σ). But for denoting surface tension lowercase sigma symbol is used.
Examples for Surface Tension:
Trampoline.
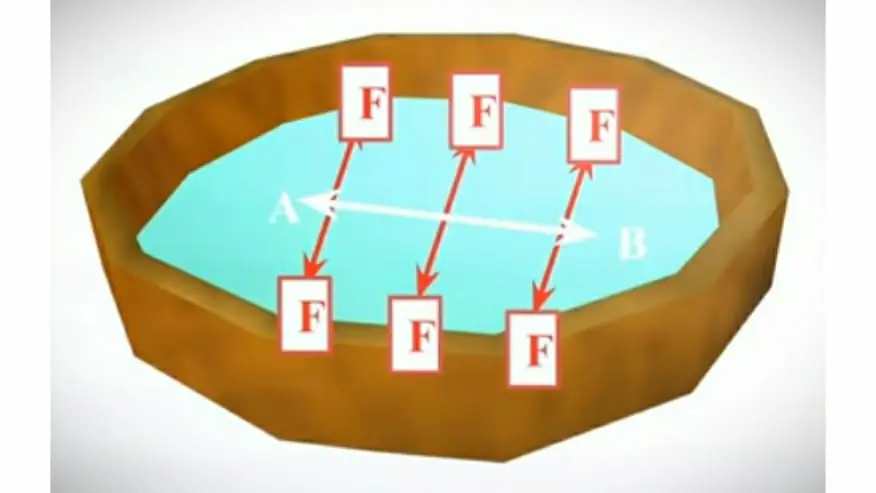
So let’s understand this definition in detail by an example. Let’s take a liquid in a bowl. Now draw an imaginary line ‘AB’ on the liquid surface. As seen in the definition surface forces act in the perpendicular or at a right angle to an imaginary line these forces are named as ‘F’.
So these surface forces you can clearly see in the diagram. These forces stretch the surface of the liquid in a stretched membrane. Due to these forces, the surface of the liquid remains in tension. This tension on the liquid surface is known as surface tension.
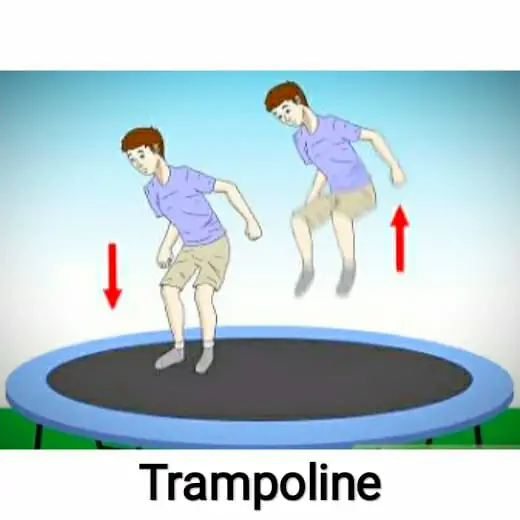
The best example of a stretched membrane is a trampoline. When you watch the surface of the trampoline, if any person jumps on the surface it tends to jump. It does not allow the person to fall on the ground. It just pushes the person back in the opposite direction.
If you observe at the corners of the trampoline there are strings attached. When a person jumps on the surface these springs stretches and due to this, the person is not allowed to fall on the ground. As similar to this example, the surface of water behaves.
The surface of the water is in tension. So due to this very light objects like pins or mosquitos can float on the surface of the water.
Effect of Impurities on the surface tension of water.
When we add impurities in the liquid or water, the nature of the bonding between the molecules changes. Due to this surface tension of water also changes. Let’s understand it in detail.
Generally, we have seen that the government sprays kerosene on the surface of accumulated water whenever there is a rise in the count of mosquitoes. It is because of spraying kerosene on the surface of the liquid. The value of the surface tension of water reduces. As the surface tension decreases the mosquitoes can not float on the surface of the water. Due to this breeding stops.
Also adding sope, phenol, as well as detergents, decreases the surface tension of water. You might have observed that when a drop falls on the surface of the cloth it does not wets the cloth, this is just because of the surface tension of water. So for washing clothes soap and detergents are used so that the surface tension of the water can be decreased.
But by adding common salt in the water, the surface tension of the water increases.
Effect of temperature on surface tension.
The surface tension of water or liquid decreases as the temperature is increased. Let’s understand it in detail.
It is generally said that, if we take warm water for washing clothes then clothes can be cleaned better than the cold water. As the temperature of the water is increased the surface tension of the water decreases.
When the temperature is increased, the average separation between the molecules increases, and due to that, the cohesive forces decrease. If we increase the temperature further the surface tension of water at one point reaches zero, this temperature is known as the critical temperature of that liquid.
Why surface tension is important?
Surface or interfacial tension is a very important phenomenon that plays a vital role in the day to day life. We can take numerous examples where surface tension plays a significant role.
If we take an example of a spider walking on the surface of the water. It is possible because of the surface tension. The surface tension of water can bear the weight of the spider. If we replace the water with ethanol, then the poor spider gets drawn into the water. It is because the ethanol’s surface tension is very low as compared to that of the water.
Surface tension is the only reason due to which the water comes down as rain in a spherical shape. Generally, the shape of the water is spherical. It is because the water tends to reduce its area. We can understand this example.
If for any given area for which an object is to be designed. Try different shapes but the smallest shaped object with the same area will be a spherical-shaped object. So due to this water always takes the spherical shape.
But now you might think that spiders are not that important, they can survive on land also and the shape of water is also not that important to worry about. But if the surface tension of the water would have been much lower than what it is at present.
Then nothing would have floated on the surface of the water. Everything, even the smallest particle on the earth would have sunken down to the bottom causing the failure of the ecosystem. Ultimately the water would have never been in the liquid form and it would have evaporated into the atmosphere and there would be no life on earth.
By now, I guess that it is very clear and I feel like you are convinced that surface tension is important for having life on the surface of the earth.
Surface tension tests.
There are three methods by which the surface tension can be measured. They are as follows:
- Capillary rise method.
- Drop method.
- Torsion balance method.
1. Capillary rise method:
To understand the concept of capillary rise let’s first understand the concept of angle of contact.
Angle Of Contact:
After the rain has stopped, you might have experienced that the drops on the leaf are round in shape or you can take the example of mercury drop on glass and in another condition, if you pour a drop of water on the glass surface then it takes a different shape. To understand this please refer to the below-given diagram.
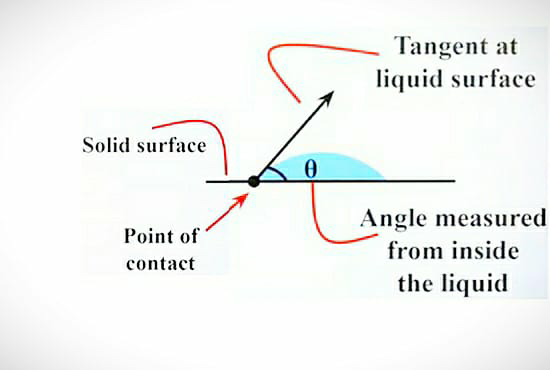
Case 1: Water drop on a glass surface
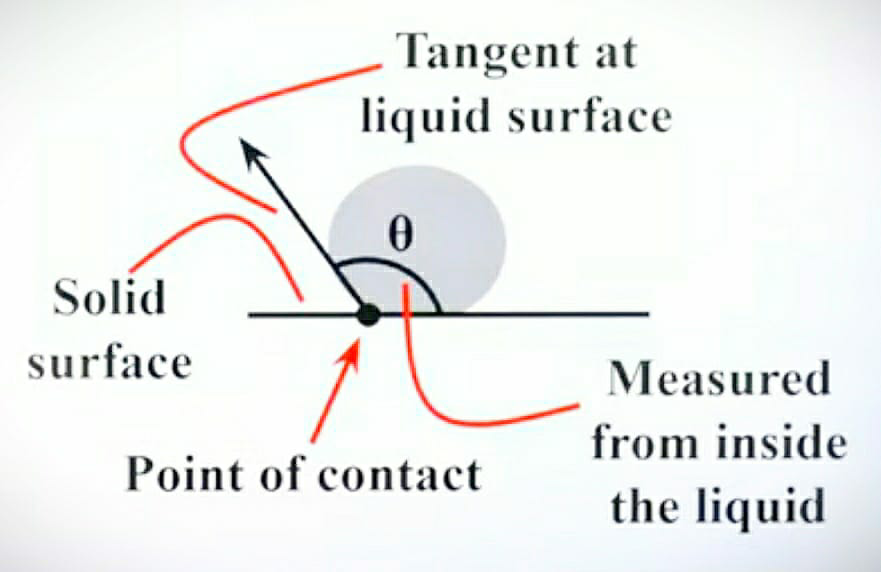
Case 2: Mercury drop on a glass surface
Case 1:
In the first case, we can see that the water droplet on the glass surface spreads nicely as compared to that of the mercury. In the case of water, the adhesion is more with respect to cohesion. In other words, we can say that adhesion dominates cohesion. So as adhesion is more in water it tries to attract or tries to stick to the surface of the glass.
So the water droplet makes this type of shape as shown in the diagram. So to study the angle of contact in case of water and glass. First, we have to draw a tangent to the water surface, and then we have to measure the angle between the tangent and the glass surface towards the water droplet side. So you will get the angle of contact of the water with glass i.e. “θ”.
Here, we came to know that when the adhesion of the liquid is more with respect to the cohesion of the liquid then the angle of contact is acute. I.e. Angle of contact is less than 90°.
Case 2:
In the second case, we can see that the mercury droplet on the glass surface does not spread as compared to that of the water. In the case of mercury, the cohesion is more with respect to the adhesion. In other words, we can say that cohesion dominates adhesion. So as cohesion is more in mercury it does not get attracted to the surface of the glass.
So the mercury droplet makes this type of shape as shown in the diagram. So to study the angle of contact in case of mercury and glass. First, we have to draw a tangent to the surface of mercury, and then we have to measure the angle between the tangent and the glass surface towards the mercury droplet side. So you will get the angle of contact of the mercury with glass i.e. “θ”.
Here, we came to know that when the cohesion of the liquid is more with respect to the adhesion of the liquid then the angle of contact is obtuse. i.e. Angle of contact is more than 90°.
Now, coming back to the topic, let’s study the capillary action method.
Capillary Action:
What is Capillarity?
Capillarity is defined as a phenomenon of rising or falling of a liquid surface in a small tube relative to the adjacent general level of liquid when the tube is held vertically in the liquid.
There are two conditions in capillary action they are as follows:
- Capillary rise.
- Capillary fall.
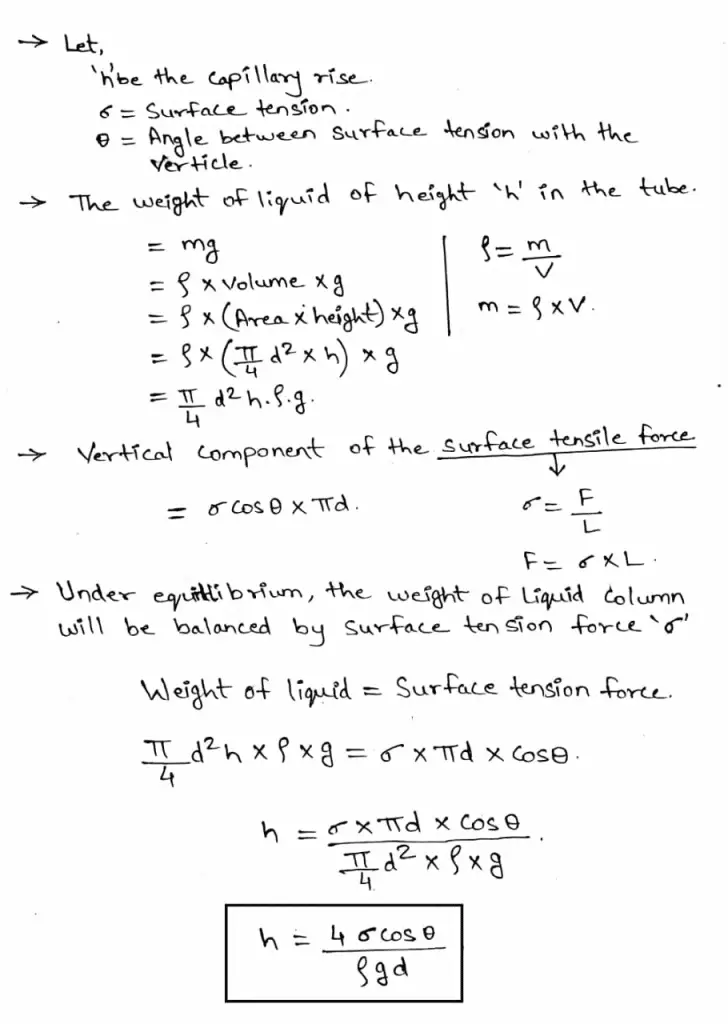
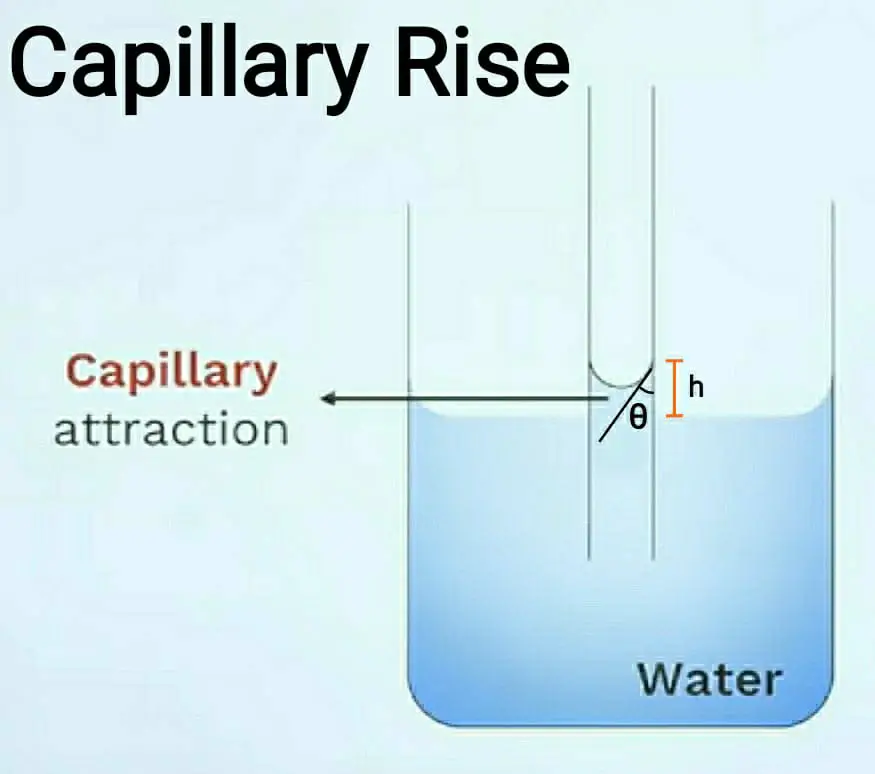
1. Capillary rise:
This condition is formed in any liquid whose adhesion is more than cohesion. So take a liquid in a beaker containing water. Then take a very thin glass tube and insert it in the liquid.
When you insert it in the liquid then the water rises by height ‘h’. When the water rises in the tube it forms a meniscus at the top, it is in a convex shape. This capillary rise is also known as capillary attraction. To measure the height ‘h’ the formula is given below and also refers to the diagram for better understanding.
2. Capillary fall:
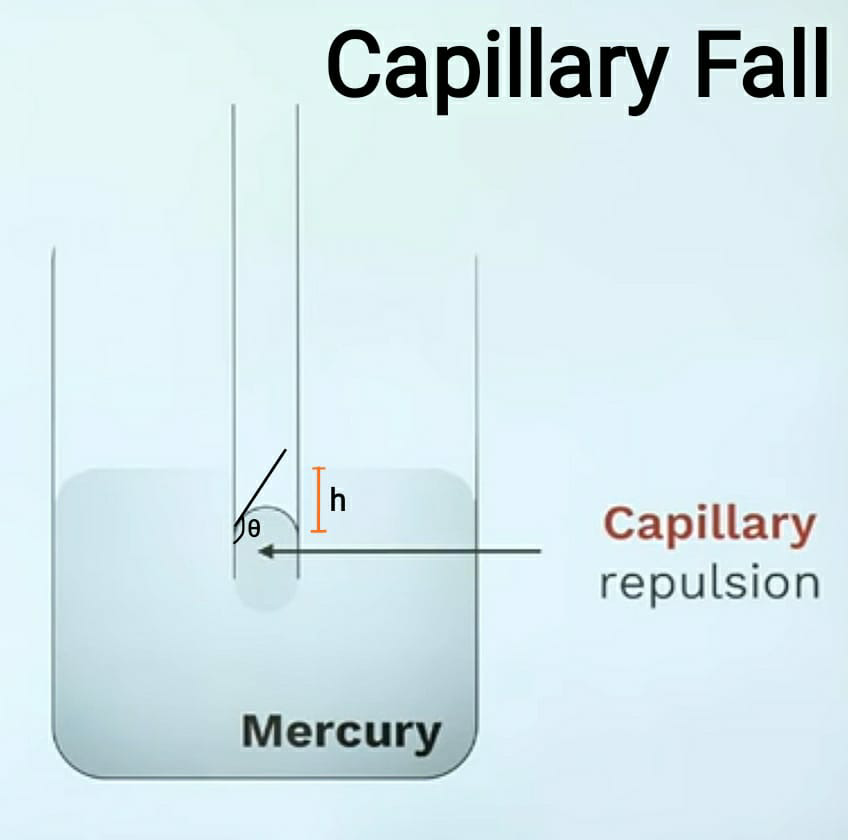
This condition is formed in liquid like mercury whose cohesion is more than adhesion. So take a liquid in a beaker containing mercury. Then take a very thin glass tube and insert it in the mercury. When you insert it in the mercury then the level of mercury falls by height ‘h’. When the mercury falls in the tube it forms a concave shape at the top. This capillary fall is also known as capillary repulsion. To understand it in detail refer below-given diagram and to measure the height ‘h’ the formula is also given below.
To calculate the value of ‘h’ you can use the following formulae:
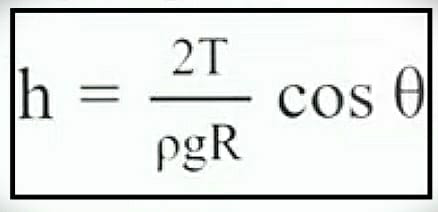
Where,
h = Height of rise or fall
T = Surface tension
g = Acceleration due to gravity
R = Radius of tube
ρ = Density of liquid
Θ = Angle of contact
2. Drop method:
We can identify the relative surface tension of any liquid by using a stalagmometer. This method is known as the drop method. This instrument has a bulb in the middle of the tube. This bulb is connected to two tubes at the top and bottom. In these tubes, two markings are given. The tube at the top is marked with ‘A’ and the tube at bottom is marked with ‘B’. Understand this tube in detail by looking at the diagram given below.
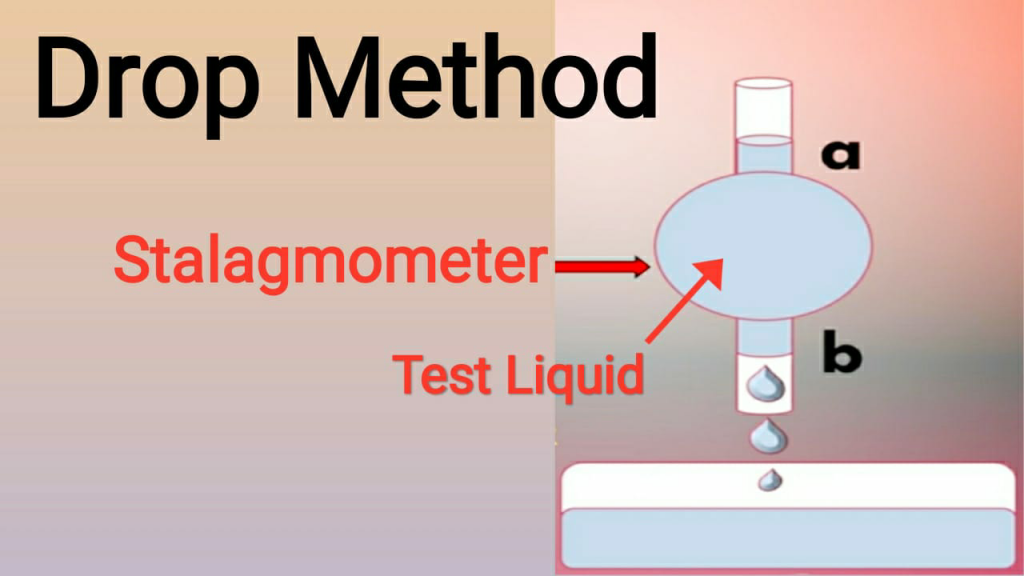
Test Process:
Initially, water is added to the tube from point ‘A’ to point ‘B’. Then the water is allowed to fall in the beaker drop by drop and the number of drops is recorded. After this water is replaced with test liquid and the same process is carried out. This procedure is carried out 3 times. To measure the relative surface tension you can use the following formula.
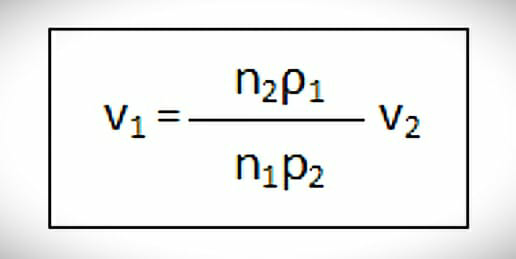
Where,
V1 = Surface tension of test liquid
V2 = Surface tension of water
ρ1 & ρ2 = Density of test liquid & water
n1 = No. of drops of test liquid
n2 = No. of drops of water
Surface Tension Measurement.
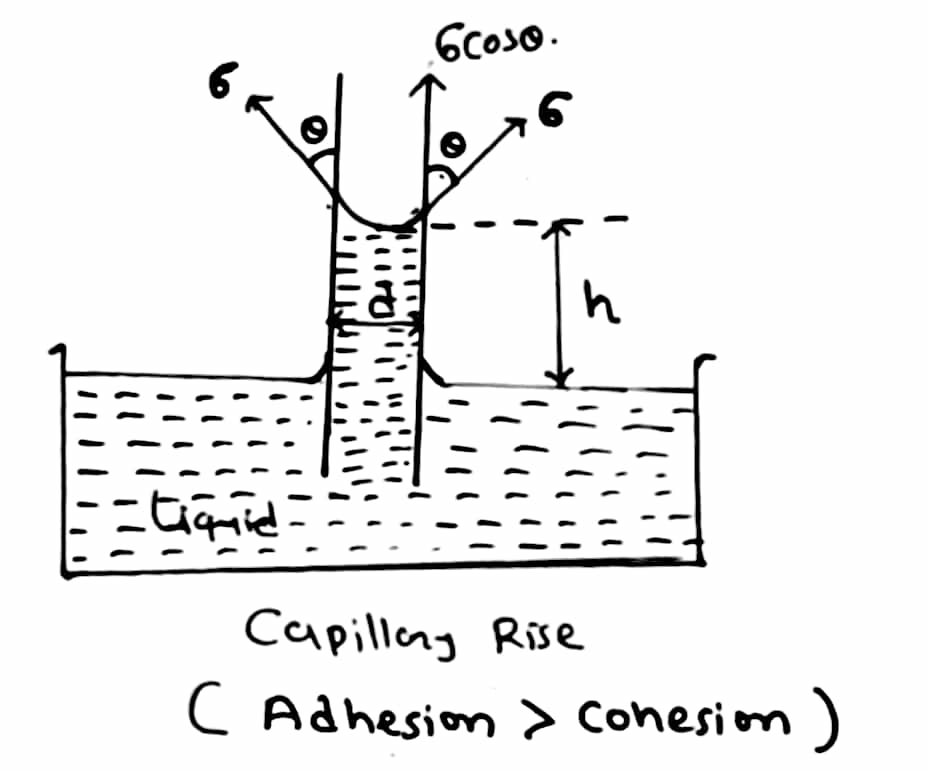
Derivation: